RSTN Trials Day 2018
The RSTN Trials Day 2018 will be on Friday 22nd June in Edinburgh. We are heading to the Royal College of Surgeons of Edinburgh.
This year we are putting surgical dogma in the spotlight. What is it? How can we challenge dogma?
Our speakers will address this this question from different perspectives – analysis of big data, innovation, research methodology and training. Read more below.
We are pleased to be holding the day in conjunction with the PLASTA Training Day. The day would not be possible without the support of BAPRAS and the secretariat.
The day will be broadcast as a webinar using Zoom. Click here to register: https://zoom.us/webinar/register/WN_ZiCtj0pdSCa1HEOgG_UbZQ
Organisers
Anna Allan
Matt Gardiner
Frank Acquaah (sponsorship)
Abhi Jain
BAPRAS: Kavita Prashar, Elizabeth Andrade, Helen Roberts. Catherine Gibbon made a huge contribution to the organisation of the day before she sadly passed away.
rstnteam@gmail.com
Why attend?
Open to all levels
Undergraduate through to consultant.
Accessible
The day is relaxed! No prior clinical trials experience is expected.
Clinical trials methodology
Learn about clinical trials methodology through interactive sessions and talks. CPD points available.
Pitch your idea
Submit a trial idea to the Vipers' Nest . Get feedback and the potential for support. There are prizes and bursaries available.
Network
It is a great opportunity to meet members of the different trials units as well as investigators with established trials.
Don't forget . . . .
The meeting is free (registration fee refunded on day). There are 6 CPD points and the food is great!
Registration
Registration is now closed.
The day will be broadcast as a webinar. Click here to register: https://zoom.us/webinar/register/WN_ZiCtj0pdSCa1HEOgG_UbZQ.
It is free and can be joined at any time.
Sponsors
We are very grateful for the support of our sponsors.
Platinum sponsor

Gold sponsors





The Vipers’ Nest
The Vipers’ Nest has four 15 minute slots for oral presentations of new project ideas. There are an additional 8 poster slots. The oral and poster prize winners will receive a certificate and book prize from Oxford University Press.
The ideas might be for a package of work leading to a clinical trial or other prospective audit. Perhaps you have an idea for a systematic review, prospective audit or RCT.
The idea remains yours. The RSTN can provide advice and put you in touch with a trials unit.
Please use the PICO format for the abstract (Population, Intervention, Comparator, Outcome).
The posters can be for either new trial ideas or updates on live projects. There is a maximum of ~250 words.
The oral and poster prize winners will receive a certificate and book prize of £100 from Oxford University Press.
We are pleased to announce that 6 bursaries of up to £100 will be available to support the travel expenses of the teams submitting successful abstracts to the Vipers’ Nest oral and poster prizes. All abstracts will automatically be considered for the bursaries.
Deadline 25th May 2018 at midnight.
Speakers

Keynote speaker: Dr Reinier Feitz
Medical entrepreneur, Plastic and hand surgeon interested in value based healthcare, exponential growth and routine outcome measurements.
Bio: Reinier received his training as a plastic and hand surgeon in Groningen Universtiy, Free University of Amsterdam, Utrecht University Medical Center, Erasmus Medical Center Rotterdam (NL), Royal North Shore and Westmead Hospital Sydney Australia. He passed both the European Handsurgery exam (FESSH), the European Plastic Surgery exam (EBOPRAS). Studied at Harvard Managing Healthcare Delivery and in 2017: Singularity University, Executive Programme (CA, USA).
Co-founder of several plastic surgery educational institutions that teach surgeons from around the globe on fresh frozen specimens. He served as board secretary of Dutch association of Plastic Surgeons from 2005-2012.
Co-founder of Xpert Clinic, a private hand surgery practice that has grown into the largest hand surgery practice of Europe with over 18 hand surgeons and over 100 handtherapists. He is privileged to treat many professional athletes and musicians. As a medical entrepreneur and hand surgeon he is a frequently invited speaker on national and international meetings. He publishes extensively in the field of hand surgery and is active as a medicolegal expert (NVMSR). He is also a board member of Equipe Zorgbedrijven.
His MTP (massive transformation purpose) is to change the incidental and ad hoc collection of patient data into a routine and democratized outcome monitoring of health data. Originally he started in 2005 with this moonshot thought and by working slowly towards this their team finally realized to have a routine outcome measurement system (Pulse) in place for each hand surgery patient. His current goal is to apply predictive and individualized medicine to the field of hand surgery.
Prof Jonathan Cook
Associate Professor
Centre for Statistics in Medicine, University of Oxford
Jonathan’s main research interest is in the design, conduct, analysis and reporting of
randomised controlled trials (particularly surgical trials). Specific areas of interest include specification of the target difference in the sample size calculation, addressing interventional expertise, and methods for improving recruitment.
He also has extensive experience in systematic reviews of randomised controlled trials and diagnostic research. He has collaborated on numerous projects including randomised trials, observational and diagnostic studies, methodological projects and systematic reviews in a variety of clinical areas (anaesthesia, cardiovascular research, obstetrics and gynaecology, ophthalmology, orthopaedics, primary care, general surgery and urology amongst others). He works with Oxford Clinical Trials Research Unit (OCTRU) and is a Deputy Director of the Surgical Intervention Trials Unit (SITU).
Jonathan holds a number of external responsibilities. He serves on and chairs Data
Monitoring and Steering committee for a number of Clinical Trials, is an associate editorship of Clinical Trials, a member of the Editorial Board of Trials, and is a statistical consultant for the British Journal of Surgery. Other responsibilities include membership of the IDEAL collaboration steering group and also of the ConDucT-II MRC Hub for Trials Methodology.
Mr Ewan Harrison
Clinical Senior Lecturer and Honorary Consultant Surgeon
University of Edinburgh
His interests focus on health informatics, clinical trials, and global surgery. He leads the Surgical Informatics group and co-leads the Surgical and Perioperative Health Research Group, performing informatics-orientated research focused on improving patient outcomes after surgery. A current collaboration with industry is joint-funded by the MRC and Kidney research UK is investigating strategies to reduce organ injury in transplantation. He has completed an MSc in Statistics. His work is currently funded by the Wellcome Trust, MRC and Academy of Medical Sciences.
Mr Simon Wood
Chair, SAC Plastic Surgery
Consultant Plastic Surgeon and Honorary Senior Lecturer, Imperial College London
Mr Simon Wood qualified in medicine at the Royal London Hospital and completed training in plastic surgery through east of England rotation in Cambridge and Norwich.
At Imperial College Healthcare NHS Trust he has developed the reconstruction service, working with breast surgeons, head and neck surgeons, colorectal surgeons and gynaecologists on a regular basis. Mr Wood has also developed the abdominal wall reconstruction service at the Trust and deals with a wide range of complex and recurrent incisional hernia problems.
In addition to surgery, Mr Wood has had a strong interest in teaching and training. In 2012 he was appointed to the Plastic Surgery Specialist Advisory Committee (SAC), part of the Joint Committee on Surgical Training (JCST) for the four Royal Colleges of Surgery. This work has included being in charge of National Selection and in 2015 he was appointed Chair of the Plastic Surgery SAC.
Mr Nick de Pennington
Deputy Chief Information Officer and Digital Officer, OUH NHS Trust
Founder and CEO, ufonia
Co-founder and MD, The Hill
Programme
The day will be broadcast as a webinar using Zoom. Click here to register for free: https://zoom.us/webinar/register/WN_ZiCtj0pdSCa1HEOgG_UbZQ
| 0900 | Registration |
| 0930 | Welcome |
| 0935 | By Plastic Surgeons, For Plastic Surgeons: An Introduction to Medical Indemnity by PRASIS Mark Henley |
| Session one: developing a trial idea | |
| 0945 | Sandpit Session Theme: Challenging dogma Aims: |
| 1100 | REFRESHMENTS, NETWORKING AND VIPERS’ NEST POSTERS |
| Session two: Methodology and Elevator updates | |
| 1130 | Keynote speaker Artificial intelligence and study outcomes Nick De Pennington |
| 1150 | Keynote speaker Placebo trials Jonathan Cook |
| 1210 | Elevator updates on current projects |
| 1210 | Update on breast reconstruction projects Matthew D. Gardiner |
| 1220 | THESEUS, Treatment of Hidradenitis Suppurativa Evaluation Study Jeremy Rodrigues |
| 1225 | TRIGGER Jeremy Rodrigues |
| 1230 | NINJA: the definitive trial Abhilash Jain |
| 1235 | An introduction to the burns audit network Saahil Mehta |
| 1245 | Keynote speaker Trainees in plastic surgery clinical trials Simon Wood |
| 1300 | LUNCH, NETWORKING AND VIPERS’ NEST POSTERS |
| Session three: Running a successful trial | |
| 1400 | Keynote speaker The use of big data in improving clinical outcomes Reinier Feitz |
| 1420 | Keynote speaker Improving patient outcomes through collaboration and clinical trials Ewen Harrison |
| 1440 | Keynote speaker PreHEAT pilot tria Saahil Mehta |
| 1450 | Discussion |
| 1500 | REFRESHMENTS, NETWORKING AND VIPERS’ NEST POSTERS |
| Session four: Vipers’ Nest – new trial ideas | |
| 1530
|
Pro-BRA – PROsthetic Breast Reconstruction Analysis: Pre-pectoral versus Sub-pectoral reconstruction – A Randomised Controlled Clinical Trial
Smeeton B & Douek M. Guy’s & St. Thomas’, London |
| 1545
|
Steroid versus placebo injection for base of thumb osteoarthritis
Dean BJF, Riley N Nuffield Orthopaedic Centre, Oxford |
| 1600
|
A prospective, randomised placebo-controlled trial investigating the benefits of pre-operative botulinum toxin A in defect closure within complex abdominal wall reconstruction
Joji N, Ross D Guy’s & St. Thomas’ Hospital |
| 1615 | Management of Extra-articular Fractures of the Fifth Metacarpal: Operative versus Non-operative Treatment (FORTE)
Wormald JCR, Claireaux H, Furniss D, Costa M NDORMS, University of Oxford |
| 1630 | Close of meeting
Certificates will be awarded on receipt of the electronic feedback forms that will be distributed after the event. |
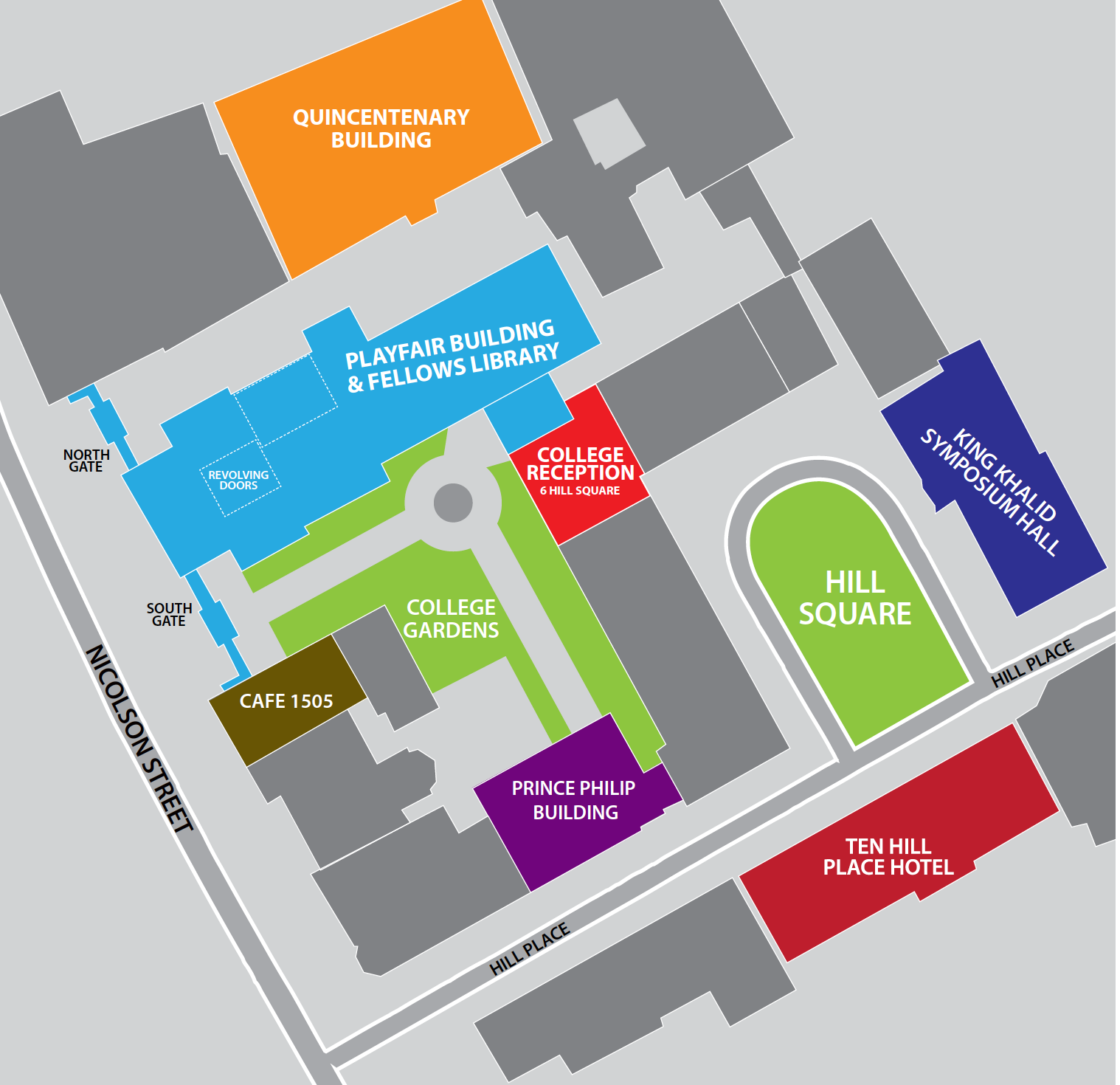
Venue
The meeting will be held in the Prince Philip Building at the Royal College of Surgeons of Edinburgh, Nicolson Street, Edinburgh, EH8 9DW.
Reception
At the end of the PLASTA day on Thursday 21st June, there will be a complimentary drinks reception with canapés in the Charter suite, Prince Philip building, Royal College of Surgeons of Edinburgh.